Dissolved Gases in Purified Water
12 mai 2023
There are dissolved gases in most purified water. What are they? How much is there? How do they get there? What effects do they have?
All purified water is produced by removing impurities from natural or municipal waters. We need, therefore, to consider the gases possibly present in these feed-waters, their fates as the waters is purified and any potential contamination with the same or other gases during or after purification.
Dissolved Gases in Natural Waters
Natural waters are, to a greater or lesser extent, in contact with air. Most of the dissolved gases are absorbed from the air plus CO2 which can come in part from soil and rocks in contact with the water. Municipal waters are obtained by treating natural waters to make them safe to drink. These processes tend to have very little effect on the dissolved gas content of the water, generally increasing their contact with ambient air and reducing the variability due to local sources.
Every atmospheric gas is in equilibrium with that gas dissolved in water. The amount dissolved in water depends on the temperature and the partial pressure of the gas in the atmosphere.
Gas Content of Natural Water
The molar concentration of a gas, i, dissolved in water in contact with air is given by:
[gasi(aq)] = Pi/KH where Pi is its volume proportion in air and KH is its equilibrium constant
Typical percentages of gases in air, their equilibrium constants with water and the resulting concentrations in water in contact with air. |
The concentrations of dissolved gases in water will vary with temperature, atmospheric pressure, and location but for water at equilibrium with air at 25°C the approximate gas content of natural waters are listed in column 4. In summary, in feed-water to purification systems, there are concentrations of oxygen and nitrogen at <10ppm levels and only traces of noble gases. The level of CO2 in the water is influenced by other factors and is discussed separately.
Effect of Water Purification on Dissolved Gases
The effects of standard water purification technologies on dissolved oxygen, nitrogen and the inert gases are negligible. These gases will pass straight through reverse osmosis membranes, UV light, micro and ultra-filters, ion exchange resins and electrodeionisation (EDI) units and do not use any of the capacity of the purification media. Any minor effects due, for example, to local changes of pressure will tend to be balanced out by equilibration with the atmosphere in any storage vessel. Feed water from municipal supplies is likely to warm as it purified in the laboratory and, as a result, the purified water is more likely to be saturated in nitrogen and oxygen.
Carbon Dioxide
Carbon dioxide behaves very differently from the other gases in air. When it dissolves in water, it reacts to set up a series of equilibria:
Equation for Dissolved CO2 in Water
CO2 + H2O ↔ H2CO3 ↔ H+ + HCO3- ↔ 2H+ + CO32-
A small fraction of the carbon dioxide dissolved in water reacts rapidly to form carbonic acid. This, in turn, partially dissociates to form hydrogen, bicarbonate and carbonate ions. CO2 will continue to dissolve until equilibrium is reached.
The situation is further complicated by the presence of carbonate and bicarbonate ions in hard natural waters. These concentrations can be relatively high, resulting in high levels of carbonic acid and dissolved CO2. In these circumstances, dissolved CO2 concentrations can be 60ppm or more.
Like other gases, CO2 will pass unaltered through filters and UV treatment, however, due to its equilibrium with bicarbonate and carbonate ions, it can, in effect, be retained on anion exchange resins. As the bicarbonate and carbonate ions are removed on the resin, more CO2 will convert to bicarbonate until none remains.
Impact of Water Purification on Dissolved CO2
1) CO2 is removed completely from water that has been purified by ion-exchange or, to a large extent, by EDI.
2) High levels of CO2 will use up a significant amount of the capacity of the ion-exchange resin bed or could overload EDI. With 25ppm CO2 in the feed-water over two-thirds of the capacity of the mixed-bed resins can be used up by the CO2.
3) With high CO2 feed waters removal of the CO2 prior to ion-exchange could increase pack lifetimes by a factor of three or more.
After Removing Dissolved Gases
After removing dissolved gases to purify the water, ultrapure water within the purifier has a resistivity of 18.15 MΩ.cm and contains sub-ppb levels of all impurities except dissolved oxygen and nitrogen.
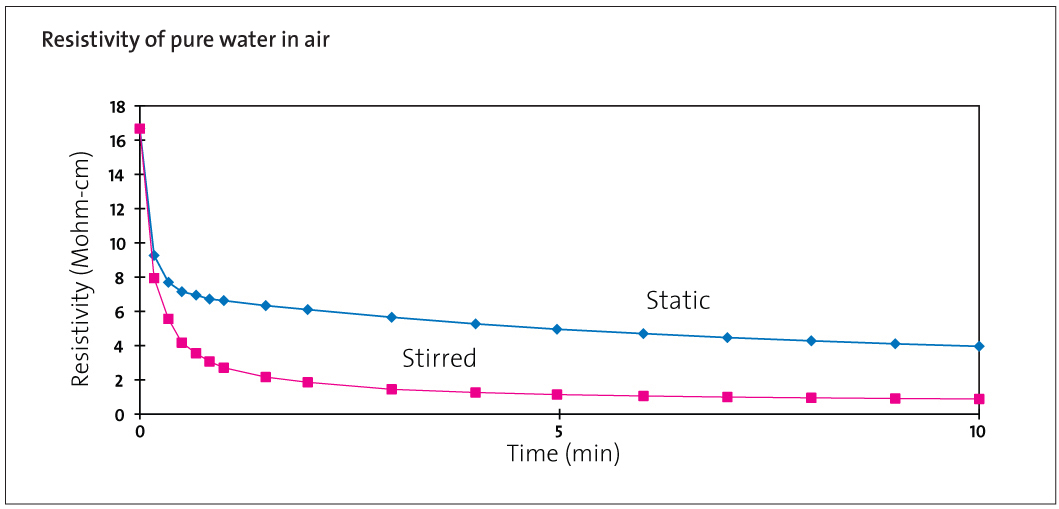
Resistivity of Pure Water in Contact with Air
As soon as this water is dispensed into a vessel in the laboratory it comes into contact with air. The oxygen and nitrogen contents do not change significantly but CO2 will rapidly be absorbed from the air; hydrogen, bicarbonate and carbonate ions will be produced and the resistivity will fall to about 1.3 MΩ.cm as shown in Figure 1. In normal laboratory practice this is unavoidable. The high conductance of the hydrogen ions means that this change only corresponds to a concentration of 0.5 mg/L CO2 in solution. This does not affect most experimentation and, with good laboratory practice, the purity of the water is otherwise unaffected.
Degassing of Oxygen and Nitrogen and Carbon Dioxide
Degassing to reduce the concentration of dissolved CO2 before ion exchange or EDI is highly desirable to increase resin pack life and ensure the effectiveness of EDI.
What is Degassing?
Degassing refers to the removal of dissolved gases for liquids, usually water or aqueous solutions. Degassing water is best carried out in the permeate from reverse osmosis (RO). At that stage, most of the ions have been removed and the pH of the water is slightly acidic; the equilibrium concentration of C02 is reduced.
For laboratory scale units hydrophobic filters are used. RO permeate flows through a bundle of microporous hollow fibers (usually polypropylene) and air sweep gas at reduced pressure flows past the outside of the fibers. The partial pressure of the CO2 in the air outside the fibers is much lower than in the water and so CO2 is removed from the water into the gas stream. Turbulence within the sweep gas makes the process more efficient but with small units, complete removal of dissolved gases is not possible. However, under suitable conditions, over 80% of CO2 can be removed with an air sweep gas.
Why is degassing important in purified water?
The removal of oxygen/nitrogen from pure water on a laboratory scale is usually done where there is concern that bubbles of gas will be released as the pressure or temperature of the water varies. This can cause blocking of fine tubes or interfere with spectrophotometric measurements due to bubbles in cells.
If the water is being degassed to reduce levels of oxygen and/or nitrogen, the same type of degasser module can be used with a vacuum applied to the outer chamber. It is best carried out on the water in the final purification loop. Oxygen and nitrogen will be reabsorbed if there is any contact with air so any such degassed water must be used via a direct link to the apparatus. For HPLC and IC this is often most conveniently achieved after water purification by sparging a reservoir with helium to remove dissolved gases.
Dr Paul Whitehead
After a BA in Chemistry at Oxford University, Paul focused his career on industrial applications of chemistry. He was awarded a PhD at Imperial College, London for developing a microwave-induced-plasma detector for gas chromatography. He spent the first half of his career managing the analytical support team at the Johnson Matthey Research/Technology Centre,specialising in the determination of precious metals and characterising applications such as car-exhaust catalysts and fuel cells. Subsequently, as Laboratory Manager in R&D for ELGA LabWater, he has been involved in introducing and developing the latest water purification technologies. He now acts as a consultant for ELGA.
