Different Types of Pure Water For The Lab: What You Need to Know
28 abr 2021

As you may know, there are several types of water purity available depending on what you’re tackling at the bench. This includes a range from Type III for general use such as rinsing out your beakers, all the way up to Type I+ for sensitive applications like the intriguingly sounding ‘graphite furnace atomic absorption spectrometry’ (GF-AAS).
A Quick Guide to Pure Water Types:
Making the decision on which type of pure water you need for your application can be challenging. Knowing this informs the purification technology you use and systems you require to produce the right water grade. See our guide on how to select the right water purity for your lab applications, where we’ve assembled a reference table for you to pick the right type of water easily and quickly.
As we’ve said, there are several different types of water, however at ELGA we specialise in the production of three main types in particular: Type I, Type II and Type III.
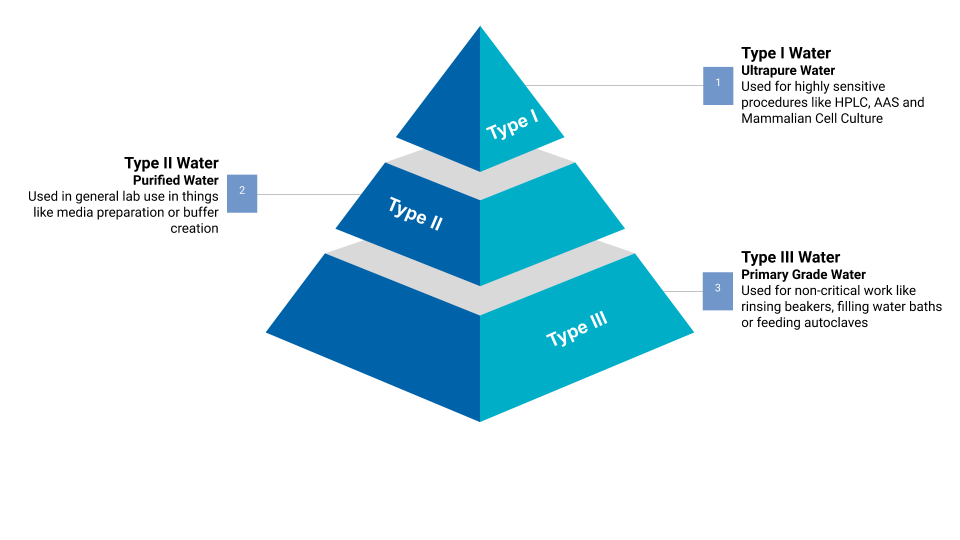
Type 1 Water (Ultrapure Water)
Type I grade water, also known as Ultrapure Water, is the purest form of water to be produced. It’s used for the most critical applications and advanced analytical procedures.
Typically, Type I water has a resistivity of 18.2 MΩ·cm and TOC levels below 10 ppb, making it suitable for sensitive applications, such as
This includes:
- Cell and Tissue Cultures
- Liquid Chromatography, including High Performance Liquid Chromatography (HPLC)
- Gas Chromatography
- Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
- Molecular Biology
Type I can also be used in applications that require Type II water. This is quite a common practice that can help to avoid the generation of by-products during applications.
Type 2 Water
Type II water grade doesn’t have the same pureness of Type I, but still maintains high levels of purity. It is a good feed water for clinical analyzers as the calcium build-up is reduced with this water type.
It can also be used in applications such as:
- General Lab Practices
- Microbiological Analysis and preparation
- Electrochemistry
- FAAS
- General Spectrophotometry
It can also be used as feed water for Type I water production.Explore your options for a type II lab water system with our PURELAB Chorus 2.
Medical Grade Water
Medical grade water meets strict standards and must be sterile and pyrogen-free to ensure safety and efficiency in medical applications. Though not the same as Type I (the highest purity), this can be used in place of medical grade water
Type 3 Water (RO Water)
Type III grade water, also known as RO water, is water produced through the purification technology reverse osmosis. Of all the pure water types it has the lowest level of purity, but is typically the starting point for basic lab applications, such as cleaning glassware, heating baths or media preparation. It can also be used as a feed water for Type I water production.
How is Laboratory Water Purity Assessed and Defined?
To implement a coherent classification system for water purity, we make use of several key factors describing the various properties of water.
The Conductivity of Water
Conductivity is reported as microSiemens per centimeter (µS/cm) at 25oC and is the reciprocal of resistivity and provides a measure of a fluid's ability to conduct electrical current. Conductivity is typically used when assessing water ranging from 'raw water' through to 'drinking water' and provides a valuable, non-specific indication of the level of ions in the water.
The Resistivity of Water
Reported as Mega-Ohms per centimeter (MO-cm) at 25oC, resistivity is related to conductivity: a high resistivity equals a low conductivity. As such, it also provides a measure of the water's ionic content. Unlike conductivity, resistivity is primarily used in the assessment of ultrapure water.
Organic Compound Levels in Water
Organic compounds can exist in water in numerous forms and so measuring every single one individually is impractical. Instead, the most useful indicator is considered to be the total organic carbon (TOC) content of the solution. This is measured via a process that oxidizes the organic compounds present and then quantifies the oxidation products generated. TOC is as close as we can currently get to a 'universal indicator' for the presence of organic impurities.
Alternatively, chromatographic techniques may be employed to determine the specifics of organic content, but this is frequently considered both too expensive and time-consuming to be used in general monitoring workflows.
Biological Contamination of Water
The presence of biological contaminants such as bacteria and other microorganisms is a common issue in untreated water. Bacterial levels reported as colony forming units per milliliter (CFU/ml) are kept low via filtration, UV treatment and sterilant solutions.
Following an incubation period in suitable growth media, individual bacterial species and total viable cell counts can be determined. Bacteria counts may also be monitored through the use of epifluorescence testing to rapidly detect and distinguish between dead and living microorganisms.
In addition to the bacteria themselves, endotoxins produced from the cell wall of gram-negative microorganisms (reported as endotoxin units per milliliter, EU/ml; 1 EU/ml approximately equal to 0.1 ng/ml) can be assessed using standard tests based on Limulus Amebocyte Lysate activity.
The Presence of Colloids in Lab Water
Suspended particles can cause water turbidity (measured in Nephelometric Turbidity Units, NTU) and are therefore filtered out of laboratory water as much as possible. This colloidal material is defined as being less than 0.5 µm in size and may contain iron, silica, aluminium or organic materials. The Fouling Index (FI) is frequently used to estimate the potential of water to block filters under 0.45 µm filter conditions.
Purification Technologies
There is a variety of methods used to achieve and maintain each water type, such as:
- Reverse osmosis
- Deionisation
- Ultrafiltration
As mentioned above, Type 3 water is RO treated. Type 2 water typically is produced via RO and deionisation. Type 1 water has further deionisation, organic degradation typically through oxidation, and bacterial removal typically through UV radiation. Ultrafiltration can be used to remove particles and larger organics from any type of purified water.
Boards Setting the Standards of Water Purity
There are several international boards around the world that have been working to establish some degree of consistency in the standards of water purity – the more people we have agreeing to these standards, the easier it is to generate reproducible data. Some labs will also adopt standards as outlined by the regulatory body overseeing the region they work in, for example, as found in the European, US or Japanese Pharmacopoeias. However, very few of these standards are specific to a particular application.
Clinical and Laboratory Standards Institute (CLSI) – formerly NCCLS
As of 2006, the CLSI has moved away from the typical Type I, II and III designations, instead preferring to suggest that water be simply ‘fit for purpose’, and only describes one grade in significant detail: Clinical Reagent Laboratory Water. The CLSI has also briefly outlined other grades in less detail, such Special Reagent Water (SRW) and instrument feed water.
International Organization for Standardization (ISO)
The ISO based its specification on ISO 3696:1987, and specifies three grades of water: Grade 1, Grade 2 and Grade 3, where Grade 1 is the most pure (see below):
Water quality parameters for ISO grades
Parameter | Grade 1 | Grade 2 | Grade 3 |
pH value at 25oC | – | – | 5.0–7.0 |
Conductivity (μS/cm) at 25oC, max | 0.1 | 1.0 | 5.0 |
Oxidisable matter Oxygen content (mg/l), max | – | 0.08 | 0.4 |
Absorbance at 254 nm and 1 cm optical path length, absorbance units, max. | 0.001 | 0.01 | – |
Residue after evaporation on heating at 110oC (mg/kg), max | – | 1 | 2 |
Silica (SiO2) content (mg/l), max | 0.01 | 0.02 | – |
American Society for Testing and Materials (ASTM)
The ASTM uses D1193-06 and has four grades of water (see table below).
Water quality parameters for ASTM types
Parameter | Type I* | Type II** | Type III*** | Type IV |
Conductivity (μS/cm) at 25oC, max | 0.056 | 1.0 | 0.25 | 5.0 |
Resistivity (MΩ-cm) at 25oC, max | 18.0 | 1.0 | 4.0 | 0.2 |
pH at 25oC | – | – | – | 5.0–8.0 |
TOC (μg/l), max | 50 | 50 | 200 | No limit |
Sodium (μg/l), max | 1 | 5 | 10 | 50 |
Silica (μg/l), max | 3 | 3 | 500 | No limit |
Chloride (μg/l), max | 1 | 5 | 10 | 50 |
*Requires use of 0.2 μm membrane filter; **Prepared by distillation; ***Requires the use of 0.45 μm membrane filter.
As you can see, understanding choosing which pure water type you will use could quickly get confusing!
Thankfully, when you choose an ELGA water purification system, you can rely on it to deliver the level of purity you need, whether that is Type 1, 2 or 3 water.
Check out our case studies section to see how ELGA LabWater has been used in different real-life laboratory settings.
